52+ calculate the empirical formula of the hydrocarbon.
Molar ratio of C. Web The empirical formula of a hydrocarbon is CH2 and its Mr is 42.

Empirical Formula Of A Hydrocarbon Mr Pauller Youtube
Web 026811 moles of H2O since there are2 moles of H in 1 moles of H2O.

. Calculate the empirical formula of the hydrocarbon that contains 9375 carbon and 625 hydrogen 70853m0162007 -1258 5 2011 gC. And thus you take a known mass of hydrocarbon. Web A hydrocarbon is found to contain 48 g of carbon and 10 g of hydrogen.
Web Calculate the empirical formula of the hydrocarbon. M CO ₂ 3301 g. The empirical formula of the hydrocarbon is CH ₂.
CH2 The foul odor of rancid butter is due largely to butyric acid a compound containing carbon hydrogen and oxygen. Web A hydrocarbon contains 20 hydrogen by mass. Web EXPERIMENT QUESTIONS 93.
H 075006. I used these molar. Ar of C 12 Ar of H 1 The action at step 5 usually gives you the.
I was asked to find the empirical formula of the hydrocarbon. Web From your empirical formula and your experimental value for the molar mass calculate the true formula. Web Determining an Empirical Formula from Combustion Data.
An organic compound contains 8647 carbon and 633. Web It is obtained on simplification of molecular formula or by the percent composition of a compound. The mass of the atoms in the empirical formula is 14 42 14 3 multiply the numbers in the empirical formula.
Aromatic hydrocarbons also known as. 053621 moles of H or 0540 g of H. Choose the type of.
Calculate the empirical and molecular formulas of this hydrocarbon. N CO₂ m CO₂ M CO₂. C ₓHₐ O₂ xC a2H₂O.
1 Molar mass of hydrocarbon melو 23 ک ۱۰ Calculate the. Web Answer is. To determine the empirical formula of hydrocarbon compound by analyzing carbon dioxide and water from a.
Calculate its empirical formula. Web Empirical Formula of a Hydrocarbon - Mr Pauller - YouTube 000 317 Empirical Formula of a Hydrocarbon - Mr Pauller Noel Pauller 646K subscribers 18K. Web The combustion analysis calculator will help you find the empirical and molecular formula of C H O compound or for a hydrocarbon.
Web Calculate the empirical formula of the hydrocarbon that contains 9375 carbon and 625 hydrogen. Web Web Find the empirical formula of the hydrocarbon H 1C 12 Complete combustion of 830 g of a hydrocarbon produced 265 g of CO2 and 950 g of H2O. The empirical formula of the compound is.
Web Separately the molar mass of this hydrocarbon was found to be 20435 gmol. Web Thus the empirical formula for the compound is CH_2 which may not be its molecular formula. Web Combustion analysis of a hydrocarbon produced 3301 g CO 2 and 484 g H 2 O.
To determine empirical formula of hydrocarbon having.

What Is The Empirical Formula Of The Hydrocarbon Cxhy That Produced The Mixture Of Products In The Diagram After A Combustion Reaction
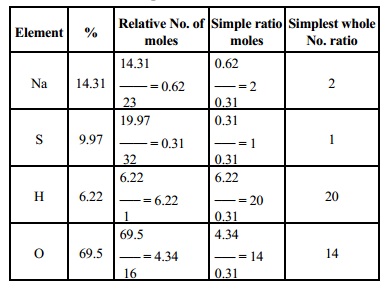
Calculation Of Empirical Formula From Quantitative Analysis And Percentage Composition

A Hydrocarbon Contains 80 Carbon What Is The Empirical Formula Of The Compound Youtube

Bismuth Atoms In Hydrocarbon Ligands Bismepines As Rigid Ditopic Arene Donors In Coordination Chemistry Organometallics
Determine The Empirical Formula Of This Compound Working Correct To One Decimal Place Sarthaks Econnect Largest Online Education Community

Empirical Formula Calculator

Empirical Formula Of A Hydrocarbon Mr Pauller Youtube

Empirical Formula Of A Hydrocarbon Mr Pauller Youtube

Predicting The Mechanism And Products Of Co2 Capture By Amines In The Presence Of H2o The Journal Of Physical Chemistry A

2 00 Grams Of A Straight Chained Hydrocarbon Contains 1 68grams Of Carbon The Rest Hydrogen 1 Calculate The Empirical Formula Of This Hydrocarbon Socratic

Coordination Chemistry 1986 By Fred Basolo Ronald C Johnson Pdf Coordination Complex Atomic Orbital

Spin States And Other Ligand Field States Of Aqua Complexes Revisited With Multireference Ab Initio Calculations Including Solvation Effects Journal Of Chemical Theory And Computation

Chemistry 4th Edition Sample Isbn 9781921917226 By Ibid Press Issuu

What Is The Empirical Formula For A Hydrocarbon Consisting Of 83 3 By Mass Of Carbon Has A Relative Molecular Mass Of 86 Quora

What Is The Empirical Formula Of The Hydrocarbon Cxhy That Produced The Mixture Of Products In The Diagram After A Combustion Reaction
Section 6 5 Emperical Versus Molecular Formulas

When A Hydrocarbon Was Completely Burnt In Oxygen 4 2g Of Carbon Dioxide And 1 71 G Of Water Were Formed Determine The Empirical Formula Of The Hydrocarbon H